Bacteria Are Destroyed When They Land On This Deadly Metal Surface Textured Using Laser
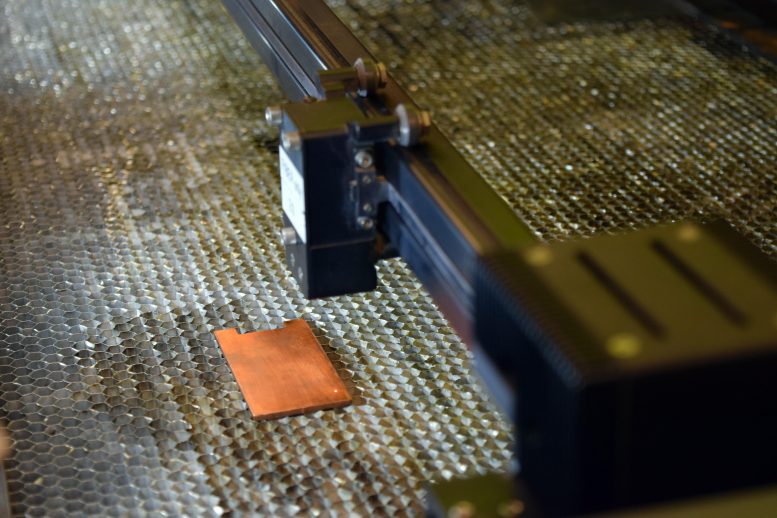
Metal surfaces and bacteria have a love and hate relationship. While bacteria easily live on some metal surfaces, they die on others. And interestingly, which metal surface they will survive on doesn’t just depend on which metal it is, but also on what the texture of the metal surface is. In other words, whether the surface is just plain and smooth, or has undulations like mountains and valleys. Recently, researchers have developed a technique in which a laser changes the metal surface from smooth to one with undulations, and when bacteria are deposited on this undulating surface, they die. In this post, I will describe the reasons behind this weird phenomenon and what its applications are.
Copper Kills Bacteria In Multiple Ways
While other metals too have bacteria killing properties, copper has been known for its antibacterial properties for millennia. While the exact mechanisms behind copper’s antibacterial properties are still being researched, there are some possible mechanisms that researchers suspect. Firstly, copper, like many other metals, easily gets oxidized. In other words, copper tends to lose electrons from its outermost shell. This is what makes metals like copper and iron prone to rusting when they come in contact with water. One of the compounds formed during rusting of copper is cuprous oxide or Cu2O. Cu2O, is rather unstable, and tends to disrupt other molecules.

When bacteria come in contact with copper, the process of rusting of copper damages the Bacteria’s own molecules on its cell surface, like phospholipids, membrane proteins and peptidoglycans of the bacterial cell wall. This process damages the cell surface of bacteria, leading to leaking of the cell contents out of it and leaking of outside molecules into the cell. This is one reason why copper surface is lethal to bacteria.
The second reason behind lethality of copper is that once the damage done by rusting causes the bacterial cell’s surface to become leaky, cupric ions, or Cu2+ ions from copper surface, leak into the cell in large numbers. Cu2+ ions can disrupt the molecular structure of biomolecules like proteins, further damaging the bacterial cells from the inside. They can also disrupt respiration and DNA replication in bacteria.
The third way in which copper damages bacterial cells is that when copper reacts with molecules in the bacteria, it leads to formation of free radicals, or chemicals that have one unpaired electron. The presence of an unpaired electron makes free radicals extremely unstable, making them react with almost any molecule around them. This causes the free radicals to damage biomolecules inside the bacteria.
In fact, it is due to these properties of copper, that chemicals like copper sulfate are toxic to humans as well, as they release Cu2+ ions in large number. Normal copper metal however, is perfectly harmless to humans, as the amount of Cu2+ ions it releases is way too low to be harmful to humans. And while copper is particularly lethal to bacteria, other metals like iron and its alloys can also be bactericidal. After all, the tendency to donate electrons and therefore produce oxides of themselves is something all metals have.
So What Does The Texture Of Metal Surface Have To Do With It?
It turns out that a big factor in deciding how toxic a metal surface like that of copper is to bacteria is the surface area of the metal surface that the bacterial cell comes in contact with. And this isn’t surprising. After all, the larger the area of the metal surface in contact with the bacterial cell, the larger will be the extent to which it will damage the cell using the mechanisms I mentioned above. And as you might guess, a rough metal surface with undulating features like microscopic mountains and valleys has far larger surface area than a smooth surface of the same metal. And this is where etching of metal surface using lasers comes into play.
Researchers at Purdue university have used very precise laser to burn the smooth surface of metals such that it becomes rough with undulations, drastically increasing its surface area. When bacteria come in contact with this metal surface, the much larger area of metal in contact with them drastically reduces their survival when compared to smooth metal surface.
This approach has multiple uses. Firstly, it can be used to make metal surgical instruments inherently deadly for bacteria, making their sterilization much easier. Secondly, many metal objects, like door knobs and handles in public transport vehicles, are hotpots of disease transmissions during a pandemic, as they are touched by multiple people every day. Texturing of these metal objects to make them bactericidal could go a long way in slowing down the spread of a future pandemic.
So far, the researchers have only made textured metal surfaces that kill bacteria, but not viruses, that are way smaller and therefore might not come in contact with large enough metal area even if the metal surface is rough. Consequently, this approach won’t work against the current COVID19 pandemic, which is caused by the SARS-CoV-2 coronavirus (covered by me in this previous post). But in near future, researchers will attempt to texture metal surfaces in such a way that the surface features cause large enough metal areas to contact viruses as well, and hopefully, it would destroy the viruses.
Another advantage of textured metal surfaces is that since they are a physical rather than chemical method of attack, its very unlikely that the bacteria will develop resistance against them like they do against antibiotics. Although a few instances of copper resistant bacteria are known. And a yet another advantage is that while excessive use of antibiotics is harmful to humans, these textured metal surfaces are not harmful to humans. The reason behind this is currently not clear. But its likely that it has something to do with surface area.
The features the researchers have created on metal surface have dimensions comparable to the size dimensions of bacterial cells, but they are way too large compared to the size dimensions of viruses and way too small compared to the size dimensions of eukaryotic cells that make up the human body. That’s likely the reason why neither the viruses nor the human cells come in contact with an area of this textured metal surface large enough to harm them.
Moreover, while metal alloys like steel are normally not as lethal to bacteria as copper, with increased surface area using texturing, they too can turn bactericidal. And since steel is used far more than copper in objects like handles, door knobs and surgical instruments, this matters a lot in determining how useful this technology is going to be.
Surface Area Governs Our Life Beyond Metal Surfaces
Surface area doesn’t just decide how lethal metal surfaces are for bacteria. Surface area, in fact, decides everything from how reactive a chemical mixture is to how efficiently you breathe or absorb food. An excellent example of vital role of surface area is seen in how jet fuel doesn’t ignite in a container, even when you put a burning matchstick in it, as you see in the video below. Other petroleum derived fuels like kerosene, diesel and petrol don’t fair much better.
But all these fuels burn very well even on coming in contact with a spark, when they are sprayed on the spark. This is because combustion is a surface phenomenon. It takes place at the interface between the fuel and oxygen rich air. So more the area of this interface, more will be the chance of combustion happening. And since fuel is divided into many small droplets in a spray, its surface area in a spray is orders of magnitude greater than its surface area when kept in a jar, drastically increasing its chances of combustion.
Another example of how reactive increased surface area can make an otherwise benign substance is seen in saw dust explosions. As is explained in the video below, the drastically increased surface area of wood, when it exists in the form of saw dust instead of intact blocks of wood, turns wood from something that would have slowly burned like in a campfire, into something that can literally blow up a building. The metal catalysts that are used in chemical reactions, be they nickel, copper, silver or any other metal, are also used in powder form instead of metal blocks or plates. This is because chemical catalysis is also a surface phenomenon, and powder form maximizes surface area of metals.
Finally, increased surface area is responsible for life itself. You look at biological systems, and you will find countless examples of systems striving to maximize surface area without having to occupy too much space. mitochondria and chloroplasts have highly undulating surfaces inside them (called thylakoids in case of chloroplasts). These undulations vastly increase the surface area that these organelles need to carry out respiration and produce energy rich ATP molecules, upon which the life of a cell depends. You can see the undulations in the inner surface of mitochondria in the transmission electron microscopy image below.
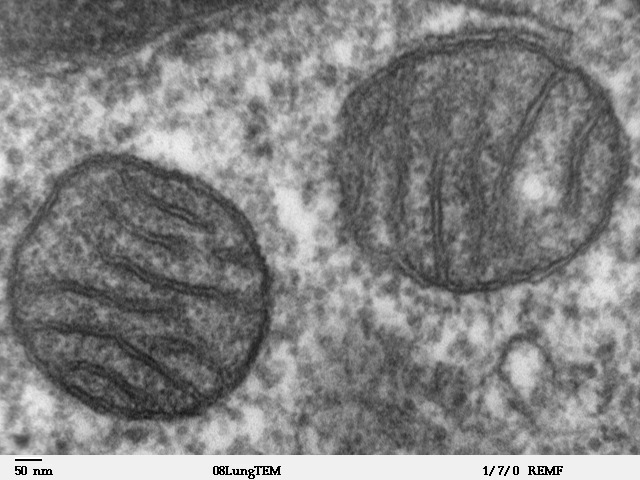
Similarly, the undulating inner surface of lungs ensure that despite being compact enough to fit in your chest cavity, they have surface area the size of a tennis court, which helps them maximize absorption of oxygen from air. The undulations in the inner surface of your intestines (called villi and microvilli), maximize the surface area of intestines, which in turn maximizes food absorption. And the disk like shape of red blood cells maximizes their absorption of oxygen. Hence, realizing that the bactericidal action of metals like copper is also a surface phenomenon, researchers have used increased surface area of metals capable of contacting bacterial cells, to transform metals from benign substances to efficient bacteria killers.
Related Posts
Testing For Coronavirus: 2 Things You Absolutely Need To Know About How Its Done
This New Method Of Treating HIV AIDS Could Finally Lead To Curing Of The Disease
SARS-CoV-2: Know The Coronavirus Strain Behind The Devastating COVID19 Pandemic
About Author
Prranshu
I am a researcher by training. I did my masters in medical biotechnology and am currently doing a PhD in biochemistry from the M. S. University of Baroda. Follow me on Twitter @PrranshuYadav.